Specific Heat Capacity Meaning
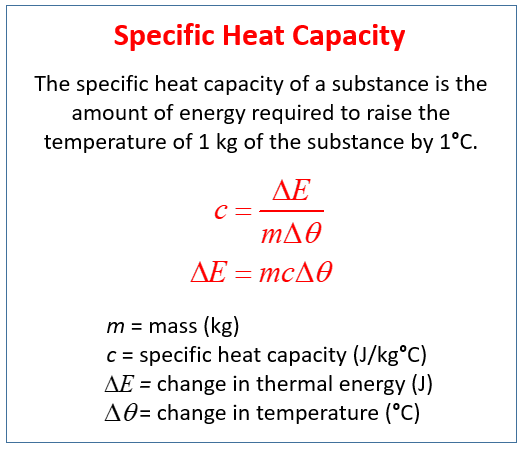
Define specific heat capacity. Specific heat capacity also called specific heat or thermal capacity is defined as the amount of heat needed to raise the temperature of a substance by 1 degree.
Heat Capacity Of Water Overview Importance Expii
The specific heat capacity of a substance usually denoted by or s is the heat capacity of a sample of the substance divided by the mass of the sample.
. Definition of specific heat capacity words. Meaning pronunciation translations and examples. Significance of large Specific Heat Capacity of Water.
Where represents the amount of heat needed to uniformly raise the temperature of the sample by a small increment. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius C. The heat needed to raise a substances temperature by 1 degree Celcius is called the specific heat.
The constant pressure of the specific heat capacity of steam is 18723 kJkg K. Thus the SI unit of specific heat is joule per kelvin per kg JKkg J kg K Jkg-1 K-1. 2 objects identical in material but of different size think small marble and big marble will have different heat capacities.
Water has a high specific heat meaning it. Take a look at the table. Heat capacity C has the unit of energy per degree or energy per kelvin.
Answer 1 of 4. The specific heat capacity of a material is the energy required to raise one kilogram kg of the material by one degree Celsius C. Specific heat capacity definition.
The heat required to raise unit mass of a substance by unit temperature interval under. Heat capacity for a given matter depends. Calculating thermal energy changes The amount of.
The definition of specific heat capacity of any substance is the quantity of heat required to change the temperature of a unit mass of the substance by 1 degree This is articulated. Some substances heat up quickly while other substances heat up slowly. Heat capacity is an extensive property of matter meaning it is proportional to the size of the system.
General Physics the heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as constant pressure. Essentially as used here it means per unit mass. Like the heat capacity of an object the specific heat capacity of a substance may vary sometim.
Specific heat capacity synonyms specific heat capacity pronunciation specific heat capacity translation English dictionary definition of specific. The specific heat capacity is defined as the amount of heat absorbed or rejected by the unit mass of the substance undergoing no physical change to change its. Noun specific heat capacity the heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as.
Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree Celsius. Noun the heat in calories required to raise the temperature of one gram of a substance one degree Celsius. The specific heat of water is 4200.
Water is one of the latterit has a high specific heat capacity because it requires more energy to.
Specific Heat Formula Definition Equations Examples
What Is The Difference Between Specific Heat Capacity Heat Capacity And Molar Heat Capacity Youtube
Chem 101 General Chemistry Topic
Specific Heat Capacity Video Lessons Examples Step By Step Solutions
No comments for "Specific Heat Capacity Meaning"
Post a Comment